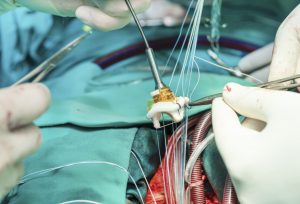
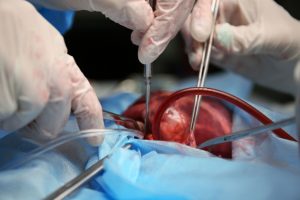
Biocompatibility and Systemic Safety of a Novel Implantable Annuloplasty Ring for the Treatment of Mitral Regurgitation in a Minipig Model
- Yuval, R., Serge, D., Nadav, Y., Udi, W., Itai, S., Avi, A., Abraham, N.,
Prosthetic annuloplasty rings are a common treatment modality for mitral regurgitation, and recently, percutaneous implantation techniques have gained popularity due to their favorable safety profile. Although in common use, biocompatibility of annuloplasty rings has been reported only sparsely in the literature, and none of these reports used the percutaneous technique of implantation. We report on the biocompatibility and the systemic safety of a novel transcatheter mitral valve annuloplasty ring (AMEND™) in 6 minipigs. This device is composed of a nitinol tube surrounded by a braided polyethylene terephthalate fabric tube. The device produced no adverse inflammatory response, showing gradual integration between the metal ring and the fabric by normal host fibrocellular response, leading to complete neoendocardium coverage. There was no evidence for adverse reactions, rejection, or intolerance in the valvular structure. In 2 animals, hemopericardium resulted from the implantation procedure, leading to right-sided cardiac insufficiency with pulmonary edema and liver congestion. The findings reported herein can serve as a case study for the expected healing pathology reactions after implantation of transcatheter mitral valve annuloplasty rings.